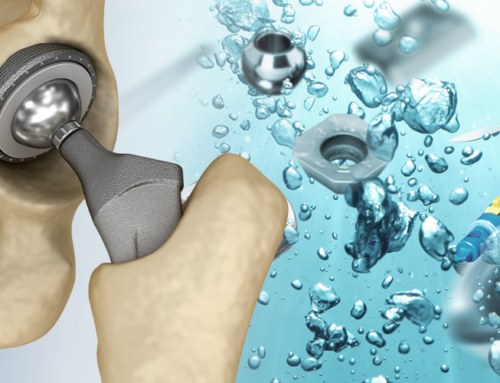
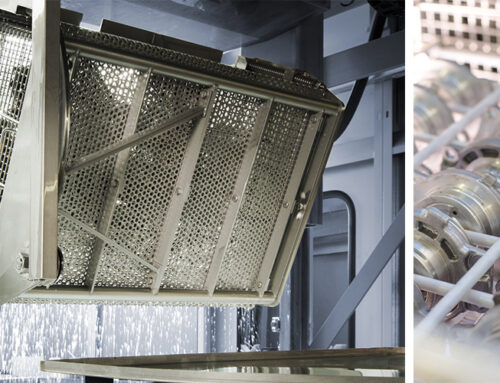
After the pre-cleaning and intermediate cleaning stages, the final step in the manufacturing process of medical implants and devices is Final Cleaning followed by Sterilization and Packaging. This step is extremely critical as it ensures that the cleaned parts are free of contamination and are ready for use in sterile medical environments.
Why Final Cleaning is Important
Even after thorough cleaning in earlier stages, components can still collect small amounts of dust, grease from handling, or light organic contamination during transfer or storage. These impurities, although not always visible, can affect:
- The sterility of the product
- Coating or bonding processes
- The safety and reliability of implants or instruments
Final cleaning is done to remove these microscopic contaminants and prepare the parts for sterilization and packaging. This ensures compliance with strict regulations like FDA (U.S. Food and Drug Administration) and MDR (Medical Device Regulation – Europe).
What Contaminants Are Removed in Final Cleaning?
This stage specifically targets:
- Fine dust
- Ambient grease or oil
- Organic contamination (fine particles)
These can get introduced during:
- Manual handling
- Visual inspections
- Transfers between cleaning and packaging areas
Suitable Cleaning Technologies for Final Cleaning
Final cleaning uses highly controlled aqueous-based processes with multi-stage immersion-type ultrasonic cleaning systems. The sophisticated Modular and customer-specific chamber systems (aqueous /modified alcohol) or our ultrasonic series immersion system (aqueous) can be adapted to any application and fine and ultra-fine cleaning requirement. This includes ultrasonic resonators for different frequencies and power levels as well as re-circulation and filtration systems. Various processes, including passivation, DI rinsing, lift-out, and drying (warm air, vacuum, IR) are applied to meet the specific task.
Machines Recommended for Final Cleaning:
- UCMBaseLine
- UCMSmartLine
- UCMPerformanceLine
These machines are designed with:
- Ultrasonic cleaning systems with Ultrasound in different frequencies and power levels
- Injection flood washing (IFW), spraying, pulsated pressure cleaning (PPC)
- Fine filtration with circular filtration systems
- Passivation, DI rinsing, lift-out, drying (warm air, vacuum, infrared, spinning)
- De-powdering (dry), powder recovery, removal of sintered powder particles
- Flexible transport system for handling of product carriers and baskets
Each machine ensures that the component meets the highest cleanliness standards required for sterile medical applications.
Typical Parts Cleaned in This Stage
Final cleaning is done for both sterile and non-sterile parts, especially:
- Orthopaedic implants (hip, knee, cranial, trauma)
- Dental and orthodontic implants
- Surgical instruments
- ENT and neuro rotary instruments
- Arthroscopic blades
- Coated components
Summary
Final cleaning is not just a process—it’s a promise. A promise that every implant or instrument sent out is safe, pure, and ready for medical use. When combined with proper sterilization and packaging, this step ensures that the patient receives a device that meets the highest global quality and safety standards.
Let us Help You Achieve Surgical-Grade Cleanliness
At Ecoclean, we understand how critical the final cleaning and packaging stages are in medical manufacturing. Our high-end cleaning systems are designed to deliver consistent, validated results—helping you meet regulatory, quality, and production goals.
Need support with your cleaning process?
Reach out to our MedTech specialists today at info.india@ecoclean-group.net